What is vibro thermography?
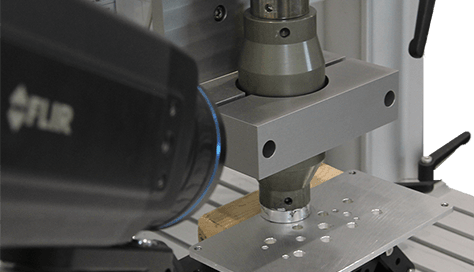
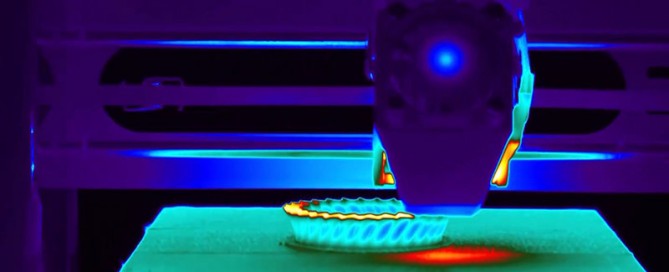
Vibro Thermography NDT Technique Vibro-Thermography is an active thermography method that uses mechanical vibration to locate cracks. It is considered an active thermography method because this method does not rely on existing heat being present in the part that is being inspected. Vibro-Thermography uses ultrasonic frequencies to excite the specimen. These ultrasonic frequencies are typically [...]